
News
Good News | Wanbangde Pharmaceutical Group’s Propafenone Fumarate Chemical API Registration Application Approved!
Date:
02 Mar,2024
Recently, Wanbangde Pharmaceutical Group Co., Ltd. received approval and issuance from the National Medical Products Administration regarding its active pharmaceutical ingredient. Propafenone Fumarate Tenofovir The "Notice of Approval for the Marketing Application of Chemical Active Pharmaceutical Ingredients."
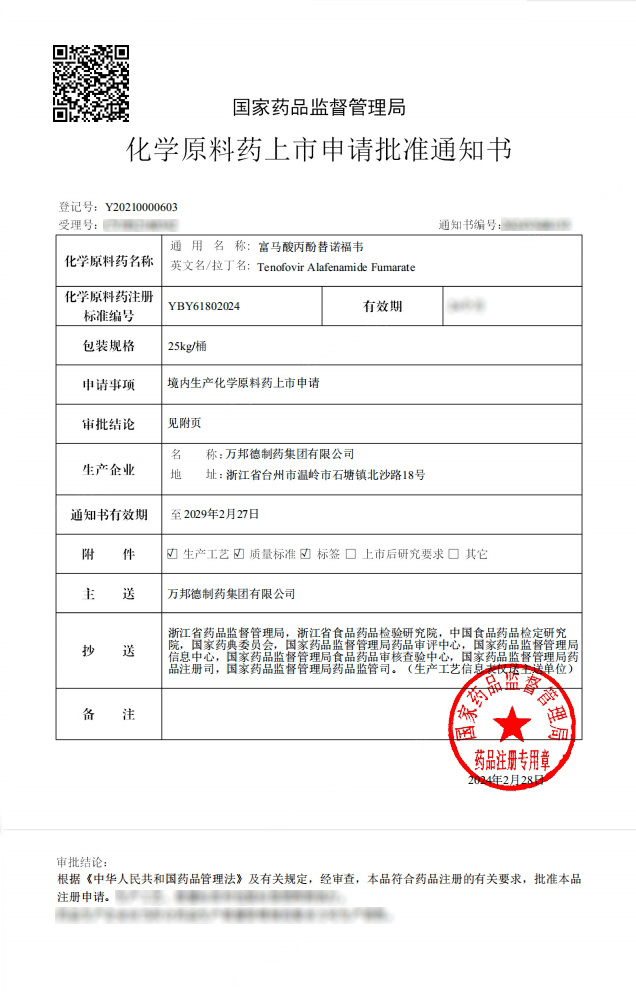
Regarding Tenofovir Alafenamide Fumarate
Tenofovir Alafenamide Fumarate (TAF) is a potent antiviral medication approved for the treatment of chronic hepatitis B (HBV) in adults and adolescents aged 12 years and older who weigh at least 35 kg. According to leading domestic and international guidelines—such as the American Association for the Study of Liver Diseases (AASLD) "Guidelines for the Prevention, Diagnosis, and Treatment of Chronic Hepatitis B (2018)," the Chinese Medical Association's "Primary Care Guidelines for Chronic Hepatitis B (2020)," and the "Chinese Guidelines for the Prevention and Management of Chronic Hepatitis B (2019 Edition)"—TAF is strongly recommended as one of the first-line antiviral therapies for managing chronic hepatitis B.
Propofol fumarate is Tenofovir Disoproxil Fumarate An upgraded version that addresses some of the limitations of tenofovir disoproxil fumarate, TAF demonstrates superior safety and efficacy compared to its predecessor. Compared to tenofovir disoproxil fumarate, tenofovir alafenamide prodrug exhibits a 5.3-fold higher intracellular drug concentration while maintaining a 91% lower plasma drug level. Unlike tenofovir disoproxil fumarate (TDF), which rapidly hydrolyzes in plasma to release tenofovir, TAF shows enhanced stability in the bloodstream and achieves significantly higher intracellular concentrations in target cells. Notably, TAF has been reported to deliver more potent antiviral activity at doses 10 times lower than TDF, while exerting minimal impact on renal function and bone density.
About Wanbande Pharmaceutical Group
Founded in 1970 and headquartered in Wenling, Zhejiang, Wanbangde Pharmaceutical Group is a high-tech enterprise dedicated to the research, development, manufacturing, and marketing of modern Chinese medicine, as well as chemical APIs and formulations. Over the years, the company has consistently pursued an integrated approach that combines industry, academia, and research, boasting a well-established technology transfer platform. Focusing primarily on therapeutic areas such as cardiovascular and cerebrovascular diseases, as well as neurological disorders, Wanbangde has developed a robust product portfolio characterized by its emphasis on natural herbal medicines, with cardiovascular and nervous system medications forming the core offerings, complemented by products targeting respiratory and other medical fields. The company also proudly holds two proprietary products with full intellectual property rights: Ginkgo Leaf Droplets. Huperzine A Injection 。

[References]
[1] Mills A, Crofoot G Jr, McDonald C, Shalit P, Flamm JA, Gathe J Jr, Scribner A, Shamblaw D, Saag M, Cao H, Martin H, Das M, Thomas A, Liu HC, Yan M, Callebaut C, Custodio J, Cheng A, McCallister S. Tenofovir Alafenamide Versus Tenofovir Disoproxil Fumarate in the First Protease Inhibitor-Based Single-Tablet Regimen for Initial HIV-1 Therapy: A Randomized Phase 2 Study. J Acquir Immune Defic Syndr. 2015 Aug 1;69(4):439-45. doi: 10.1097/QAI.0000000000000618. PMID: 25867913.
[2] Ray AS, Fordyce MW, Hitchcock MJ. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus. Antiviral Res. 2016 Jan;125:63-70. doi: 10.1016/j.antiviral.2015.11.009. Epub 2015 Nov 27. PMID: 26640223.
[3] Li X, Tan XY, Cui XJ, Yang M, Chen C, Chen XY. Pharmacokinetics of Tenofovir Alafenamide Fumarate and Tenofovir in Chinese Individuals: Effects of Non-Genetic Factors and Genetic Variations. Pharmgenomics Pers Med. 2021 Oct 14;14:1315-1329. doi: 10.2147/PGPM.S329690. PMID: 34703277; PMCID: PMC8525415.
[4] Wu Xiaoyan, Yu Hao, Zhou Yang, et al. A Meta-Analysis on the Effects of Tenofovir Alafenamide Fumarate and Tenofovir Disoproxil Fumarate on Lipid Profiles[J]. Journal of Adverse Drug Reactions, 2021, 23(11):584-591. DOI:10.3760/cma.j.cn114015-20211013-01064.
Related Recommendations
